
|

|
NINTH INTERNATIONAL PHARMACEUTICAL COMPLIANCE CONGRESS
May 11 - 13, 2015
|
ATTEND ONSITE
|
WEBCAST ATTENDANCE
|
Crowne Plaza Brussels -
Le Palace
Brussels, Belgium |
In your own office or home live via the Internet with 24/7 access for 6 months
|
|

|
|
KEYNOTE SPEAKERS
|

Robert Barrington, PhD
Executive Director, Transparency International-UK, London, UK |

Richard Bergström
Director General, European Federation of Pharmaceutical Industries and Associations (EFPIA), Former Director General, LIF Sweden, Brussels, Belgium
|

Marc de Garidel
Chairman and Chief Executive Officer, Ipsen, Vice President, European Federation of the, Pharmaceutical Industries and Associations (EFPIA), Knight, National Order of the Legion of Honor (France), Paris, France |

Brendan Shaw, PhD
Assistant Director General, International Federation of Pharmaceutical, Manufacturers and Associations (IFPMA), Former Chief Executive, Medicines Australia, Geneva, Switzerland
|
|
|
|
FEATURING AN EFPIA STAKEHOLDER WORKSHOP (Registration Complimentary)
|
 The European Federation of Pharmaceutical Industries and Associations (EFPIA) is offering an independent EFPIA STAKEHOLDER WORKSHOP from 10:30 am to 3:00 pm on Monday, May 11, 2015.
The European Federation of Pharmaceutical Industries and Associations (EFPIA) is offering an independent EFPIA STAKEHOLDER WORKSHOP from 10:30 am to 3:00 pm on Monday, May 11, 2015.
Click here for complimentary registration.
|
|
COVERING
|
- Data Privacy - Implications of the EU Regulation on Data Privacy
- Working with Patient Organisations
|
- Disclosure Code - Implementation Across Europe
- Stakeholder Perspectives on Disclosure Implementation
- Communicating Disclosure - Developing the Right Culture
|
|
FEATURING
|

Richard Bergström
Director General, European Federation of Pharmaceutical Industries and Associations (EFPIA), Former Director General, LIF Sweden, Brussels, Belgium |

Brendan Barnes
Director IP and Global Health, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium |

Nicola Bedlington
Secretary General, European Patients' Forum (EPF), Former Director, European Disability Forum, Brussels, Belgium
|

Julie Bonhomme
Legal Affairs & Compliance Deputy Director, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium |

Mieke Goossens
Legal Counsel, bij pharma.be, Brussels, Belgium |

Jens Steen Ravn Jensen, MSc
Senior Manager, Novo Nordisk A/S, Denmark
|

Krzysztof Kałużny
Project Manager, INFARMA, Warsaw, Poland |

Andrew Powrie-Smith
Director of Communications, European Federation of Pharmaceutical Industries and Associations (EFPIA), Former Director, The Association of the British Pharmaceutical Industry (ABPI), Brussels, Belgium |
|
|
|
FEATURING PLENARY SESSIONS
|
|
|
- Keynote Address: Innovation, Ethics and Compliance
- Keynote Address: The C-Suite Perspective
- European Commission Keynote Address
- Transparency International Keynote Address
- Global Chief Compliance Officer Roundtable
- Understanding the New Life Sciences Marketplace
- The New Marketplace: The Purchasers Perspective
- Roundtable on Compliance Issues Raised by the Changing Marketplace
- IFPMA International Codes Update
- Global Transparency Roundtable
- Anticorruption 2.0: Thinking Strategically/Managing Risks
- Crafting the Compliance Program of the Future and the Evolution of the Roles and Responsibilities of Compliance Professionals
- The Evolution of the Role and Responsibility of Compliance Professionals
|
INTERNATIONAL PHARMA
CONGRESS IS
|

|
|
|
|
AND MINI SUMMITS
|
- Mini Summit I: Basic Compliance Concepts and Skills I
- Mini Summit II: How to Engage Medical Societies with Conference Organizers
- Mini Summit III: Competition/Antitrust Law Update
- Mini Summit IV: Global Transparency, Disclosure and Aggregate Spend Update
- Mini Summit V: Annual Central and Eastern European (CEE) Compliance Update
- Mini Summit VI: How Transparency Initiatives Impact Life Sciences Commercial Model Evolution; Changing Relationships with Healthcare Providers
- Mini Summit VII: Basic Compliance Concepts and Skills II
- Mini Summit VIII: Global Compliance Auditing and Monitoring Best Practices
- Mini Summit VIX: What's Next in Global Compliance?
- Mini Summit X: Clinical Trials Compliance
- Mini Summit XI: Data Analytics Supporting Compliance Monitoring, Risk Prevention and Mitigation
- Mini Summit XII: Advanced Strategies in Compliance Training
- Mini Summit XIII: Annual Middle East/Africa European (MEA) Compliance Update
- Mini Summit XIV: Medical Device Compliance Issues Update
- Mini Summit XV: Advanced Issues in Anti-corruption Compliance
- Mini Summit XVI: Best Practices in Third Party Supplier Management
|
|
|
|
A CHIEF COMPLIANCE OFFICERS ROUNDTABLE
|

Martin Fürle
Vice President, General Counsel & Chief Compliance Officer, DAIICHI SANKYO EUROPE GmbH, Munich, Germany |

Thomas K. Hauser, LLM
Chief Compliance Officer, Siemens Healthcare, Board Member, ETHICS, Nürnberg, Germany |

Mwana Lugogo
Corporate Officer, Global Compliance Officer, Takeda Pharmaceuticals International GmbH, Zurich, Switzerland
|

Jeff Rosenbaum
Vice President, Chief Compliance Officer, Vertex Pharmaceuticals, Former Executive Director, Global Head of Ethics & Compliance Novartis Oncology, Boston, MA, USA |

Kris Curry
Principal, Fraud Investigation and Dispute Services, Ernst & Young LLP, Former Vice President, Health Care Compliance, Pharmaceuticals Group, Johnson & Johnson, Philadelphia, PA, USA (Moderator)
|
|
|
|
THE NEW MARKETPLACE: THE PURCHASER PERSPECTIVE
|

Günter Danner, MA, PhD
Associate Director, European Representation, German Social Insurance, Brussels, Belgium |

Klaus Hilleke, PhD
Chief Executive Officer and Head, Global Life Science Division, Simon-Kucher & Partners, Bonn, Germany |

Frank Wartenberg, PhD
President, Central Europe, IMS Health, Frankfurt/Main, Germany
|

Andrew White
Head of Medicine Management - NHS, Greater Manchester Commissioning Support Unit, Manchester, UK |

Olivier Wong, MD
Specialist in Family Medicine; Former Voting Member, French Transparency Committee, Paris, France
|
|
|
|
ANTICORRUPTION 2.0 PANEL
|

Ted Acosta, Esq.
Principal, Fraud Investigation & Dispute Services, EY, Former Senior Counsel, Office of Inspector General, United States Department of Health and Human Services, New York, NY, USA and Paris, France |

France Chain
Senior Legal Analyst, Anti-Corruption Division, Organisation for Economic Co-operation and Development (OECD), Paris, France |

Peter Dieners, Esq.
Partner and Head, Global Healthcare and Life Sciences Group, Clifford Chance, Co-chair, Legal Affairs Focus Group (LA FG), EUCOMED, Co-chair, Compliance Network (CN), EUCOMED, Düsseldorf, Germany
|

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY |

Joseph B. Tompkins, Jr.
Partner, Sidley Austin LLP, Former Deputy Chief of the Fraud Section, Criminal Division, United States Department of Justice, Washington, DC, USA
|
|
|
|
CENTRAL AND EASTERN EUROPE (CEE) COMPLIANCE UPDATE
|

Paul J. Melling, Esq.
Founding Partner, Baker & McKenzie - CIS, Limited, Moscow, Russia |

Madina Plieva, PhD
Legal Director, Association of International, Pharmaceutical Manufacturers (AIPM), Former Head of Legal Department, Kutafin Moscow State Law Academy, Moscow, Russia
|

Dumitru Uta, MD, MBA, CCEP
Ethics and Compliance Director, South-East Europe, Eli Lilly and Company, Bucharest, Romania |

Mariusz Witalis
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Warsaw, Poland (Moderator)
|
|
|
|
MIDDLE EAST/AFRICA (MEA) COMPLIANCE ROUNDTABLE
|

Flavia Bollinger
Head Integrity and Compliance MENA (Middle East, North Africa), Novartis Pharma Services AG, Dubai, UAE |

Zeynep Didem Degirmencioglu
Head of Compliance, Intercontinental Region, Merck KGaA, Istanbul, Turkey
|

Dr. Yacoub Haddad
Government Affairs Director MEAP Region, Abbvie, Chair, Pharmaceutical Research and Manufacturers Association, Gulf (PhRMAG), Dubai, UAE |

Laura Nassar
Head of Compliance, Middle East Sub-Regions I & II, Hoffmann-La Roche Ltd. (Dubai Branch), Dubai, UAE
|
|
|
|
FEATURED FACULTY
|

David Andersen
Partner, Pharmaceutical and Life Sciences Risk Assurance, PwC, London, UK |

Annalisa Ponchia Baccara, CMP, CMM
Executive Officer, European Society for Organ Transplantation (ESOT), Padova, Italy |

Brian K. Beeler, MBA, JD
Senior Vice President, Legal and Chief Compliance Officer, Horizon Pharma, Dublin, Ireland
|

Andy Bender
President and Founder, Polaris, New York, NY, USA |

Katrina Cahill
Global Transparency Lead, Biogen Idec, Weston, MA |

Fiona Carlin, LLB
Managing Partner, European & Competition Law Practice, Baker & McKenzie, Brussels, Belgium
|

Laurent Clerc
Co-founder and Regulatory Affairs Expert, BMI SYSTEM, Paris, France |

Audrey F. DeGuarde, MSJ
Director, Aggregate Spend Services, Porzio Life Sciences, Morristown, NJ |

Holger Diener, PhD
Managing Director, Association of Voluntary, Self-Regulation for the Pharmaceutical Industry, Berlin, Germany
|

Suzanne Durdevic, LLM
Senior Director, General Counsel, EMEA, Boston Scientific International; Chair, Legal Affairs Focus Group, EUCOMED; Member, Strategic Committee, ETHICS, Paris, France |

Kip Ebel, MBA
Principal, Ernst & Young, New York, NY |

Sue Egan
Director and Principal Consultant, Sue Egan Associates, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK
|

Eva Gardyan-Eisenlohr
General Counsel and Compliance Officer, Bayer Pharma AG, Member, Strategic Committee, ETHICS, Berlin, Germany |

George Fife
xecutive Director, Fraud investigation and Dispute Services, EY, Former Executive Director, Compliance and Ethics, Bristol-Myers Squibb, Former, Compliance and Internal Audit Leader, EMEA, GE Healthcare, Paris, France |

Jacques Fontas
Executive Counsel, Compliance Leader, GE, Healthcare Europe; Member, Strategic Committee, ETHICS, Paris, France
|

Dario Ghoddousi
Senior Vice President Global Transparency and MDM Solutions, IMS Health, Paris, France |

Cécile Gousset
Associate Vice President Compliance Risk Assessment, Education and Monitoring, Global Compliance, Sanofi, Member, Strategic Committee, ETHICS, Paris, France |

Ulf H. Grundmann, JD
Partner, Life Science Practice Group, King and Spalding, Frankfurt, Germany
|

Catherine Higgs, MA Cantab
Senior Legal Counsel, AstraZeneca, Alderley, UK |

Erinn Hutchinson
Partner, Advisory Services, PricewaterhouseCoopers, Philadelphia, PA |

Carl Judge
Director, Fraud Investigation & Dispute Services, EY, London, UK
|

Jimi Kehlet
Manager, Assurance intelligence, Group Internal Audit, Novo Nordisk A/S, Bagsvaerd, Denmark |

Isaure Kergall-Gayet, DEA-Law
Compliance & Business Integrity Director, France, Global Compliance & Business Integrity Department, Sanofi, Former VP, Chief Ethics & Compliance Officer, Ipsen, Paris, France |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC
|

Holly K. Kramen
Principal, Porzio Bromberg & Newman, P.C., Vice President, Porzio Life Sciences, LLC, Washington, DC |

Aline Lautenberg
General Counsel - Director Legal & Compliance, Eucomed, EDMA and MedTech Europe, Brussels, Belgium |

Angelique Lee-Rowley, Esq.
Vice President, Associate General Counsel and Compliance Officer Spectrum Pharmaceuticals, Inc., Former Director, Compliance, Siemens Healthcare, Irvine, CA, USA
|

Evelyne Lemaire
Head of Compliance, Europe & Canada, Takeda Pharmaceuticals International GmbH, Zurich, Switzerland |

Alice Lex, PhD
Health Care Compliance Officer, Janssen Research and Development in EMEA, Johnson & Johnson, Neuss, Germany |

Michael K. Loucks, Esq.
Partner, Skadden Arps LLP, Former Acting United States Attorney, District of Massachusetts, Washington, DC, USA
|

Maurits J.F. Lugard, MA, JD, LLM
Partner, Sidley Austin LLP, Former Member of the European Commission's Legal Service, Brussels, Belgium |

Robert McKague
Chief Compliance Officer, Jazz Pharmaceuticals |

Stephen Nguyen-Duc
Office of Ethics & Compliance Regional Director . International Operations, Western Europe & Canada, Abbvie, Former Director, Ethics & Compliance Officer, Member of the Executive Committee, Eli Lilly, Member, Strategic Committee Member, ETHICS, Paris, France
|

David O'Shaughnessy
Vice President, Compliance, Emerging Markets, Quintiles, Board Member, ETHICS, Former Vice President Compliance, International Pharmaceuticals, GSK, Former Vice President, Global Compliance Strategy, AstraZeneca, Reading, UK |

Pascale Paimbault
Compliance Officer, France -Benelux, Biogen, Treasurer and Strategic Committee Member, ETHICS, Former Chief Compliance Officer EMEA, Wright Medical Inc., Former Senior Director, Compliance and Ethics, Business Program, EMEA, Bristol-Myers Squibb, Paris, France |

Gregoire Pavillon
Executive Director, European Association for the Study of the Liver (EASL), The Home of Hepatology, Geneva, Switzerland
|

Oscar Perdomo
Health Industries Advisory, PwC, San Francisco, CA |

Dr. Gudula Petersen
Chief Compliance Officer Europe & Australia, Grunenthal, Aachen, Germany |

Katalin Pungor
Director, HCCO Pharma Emerging Markets, Johnson & Johnson, Budapest, Hungary
|

Nathalie Raynaud
Corporate Compliance Director, Global Compliance & Business Integrity Department, Sanofi, Paris, France |

Christian-Claus Roth
Head Global Event Governance, Novartis, Pharma; Co-President, International Pharmaceutical Congress Advisory, Association (IPCAA), Berne, Switzerland |

Guillaume Roussel
Director of Strategy, Veeva OpenKey, France
|

Fabien Roy, DESS European Law
Senior Associate, Hogan Lovells, Brussels, Belgium |

Christine Sainvil
Compliance Officer, EthicalMedTech, Brussels, Belgium |

Brian Sharkey, Esq.
Counsel, Porzio, Bromberg & Newman, Director of Compliance and Legal Affairs, Porzio Governmental Affairs, LLC, Morristown, NJ, USA
|

Heather Simmonds
Director, Prescription Medicines Code of Practice Authority; Vice-Chair, IFPMA Code Compliance Network, London, UK |

Nadine Sprangers
Senior Director Pricing, Reimbursement and Market Access, Daiichi Sankyo Europe GmbH, Munich, Germany |

Cerstin Steindorf
Global Account Director Healthcare, MCI, Geneva, Switzerland
|

Nina Stoeckel, LLM
Head of Group Compliance Programs, Merck KGaA, Darmstadt, Germany |

Michele Tagliaferri, Esq.
Counsel, Sidley Austin LLP, Brussels, Belgium |

Melda Tanyeri, MS, MBA
Director, Fraud Investigation & Dispute Services, Ernst & Young, London, UK
|

Hein Van den Bos
Counsel, Hogan, Lovells International LLP, Amsterdam, Netherlands |

Marie Vandepaer
Senior Director Compliance Awareness and Solution-Corporate Compliance, UCB SA, Brussels Area, Belgium |

Elisabethann Wright, Esq.
Partner, Hogan Lovells, Former Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|

Clara Zachmann
Ethics & Policy Manager, European Generic Medicines Association (EGA), Brussels, Belgium |

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain
|
|
|
|
2015 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
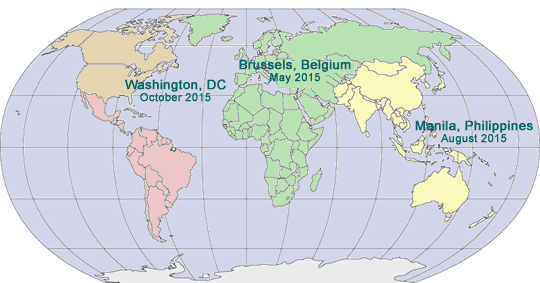
|
|
|

|
|
|
SPONSORED BY:
|
|

|
|
CO-SPONSORED BY:
|
|

|
|
CONGRESS CO CHAIRS
|

Ann Beasley
Chief Compliance Officer, Biogen Idec International, GmbH, Former Global Compliance Officer, Novartis Pharma AG, Board Member and Co-chair, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Cambridge, MA, USA
|

Dr. Michael Bartke
Director Compliance Management, DAIICHI SANKYO EUROPE GmbH, Co-chair, EFPIA Compliance Workgroup, Munich, Germany
|

Dominique Laymand, Esq.
Senior Vice President, Chief Ethics and Compliance Officer, Ipsen; President, International Society of Healthcare Ethics and Compliance Professionals (ETHICS), Paris, France
|

Roeland Van Aelst
Regional Vice President - HCCO MD&D EMEA & Canada, Office Health Care Compliance and Privacy, Johnson & Johnson, Board Member, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Chairman, MedTech Compliance Network, Brussels, Belgium
|
|
|
|
CONTINUING EDUCATION CREDITS
|
Accounting Professionals: Approved for up to 13 NASBE CPE credits.
Compliance Professionals: Approved for up to 13.0 Compliance Certification Board CCB Credits.
Attorneys:
Approved to offer a maximum of 12.50 Hours of Pennsylvania CLE Credits.
The Summit is currently pending approval to offer California MCLE Credit.
Click here for more information.
|
|
GRANTORS:
|
|
DIAMOND
|

|
|
PLATINUM
|

|
|
GOLD
|

|
|
BRONZE
|










|
|
OTHER INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai

2012 - São Paulo

2013 - Madrid, Spain

2013 - Kuala Lumpur

2014 - Dubai

2014 - Mexico City
|
|
FOLLOW INTERNATIONAL PHARMA CONGRESS ON
|


Tweet using #PharmaCongress
|
|
PARTICIPATION OPTIONS
|
|
|
|
TRADITIONAL ONSITE ATTENDANCE
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction

|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office


|
WEBCAST INTERFACE SAMPLE
|
Click here for a sample stream
|
|


This site complies with the HONcode standard for trustworthy health information:
verify here.


|
|

|

